Por favor, use este identificador para citar o enlazar este ítem:
http://hdl.handle.net/10637/6882A multiplatform metabolomic approach to the basis of antimonial action and resistance in Leishmania infantum
| Título : | A multiplatform metabolomic approach to the basis of antimonial action and resistance in Leishmania infantum |
| Otros títulos: | Análisis de Leishmania tratada con antimonio |
| Autor : | Rojo Blanco, David Canuto, Gisele A. B. |
| Autor : | Castilho-Martins, A. Tavares, Marina F. M. Barbas Arribas, Coral. López-Gonzálvez, Ángeles Rivas, Luis |
| Materias: | Leishmania infantum; Metabolomics; Antimonial action and resistance; 13C-Arginine; Multiplatform approach |
| Resumen : | Abstract: The goal of this work is to test whether a metabolomic platform, gathering different complementary analytical techniques, will improve the metabolite coverage achieved by single separation techniques, applied to the mechanism of action and resistance to Sb3+ in Leishmania infantum. This was accomplished first through the untargeted analysis of metabolic snapshots of treated and untreated parasites both resistant and responders, utilizing a multiplatform approach to give the widest as possible coverage of the metabolome, and additionally through novel monitoring of the origin of the detected alterations through a 13C traceability experiment. ------------------------------------------------------------- Resumen: El objetivo de este trabajo es comprobar los mecanismos de acción y resistencia a Sb3+ en Leishmania infantum desde un enfoque multiplataforma con técnicas analíticas complementarias y conseguir una mejora en la cobertura metabólica global. Primeramente se analizaron los perfiles metabólicos en parásitos tratados, no tratados y resistentes. Obtenidas las pertinentes conclusiones, se procedió a la monitorización del origen de las alteraciones metabólicas detectadas a través de una novedosa metodología para la trazabilidad de 13C. |
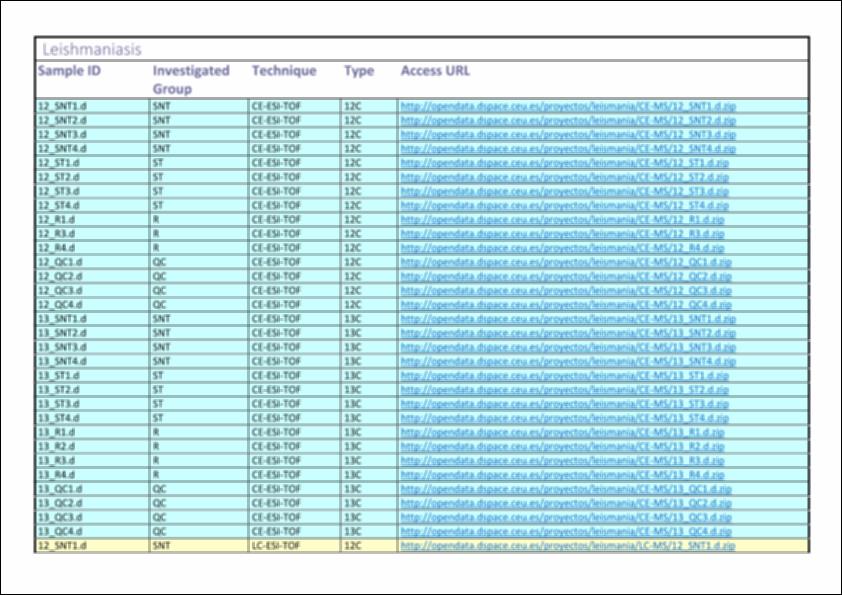
| Descripción : | Sample type: Promastigote cell cultures. Once they reached a mid- exponential phase of growth (8.000.000 promastigotes/mL) they were transferred into the same volume of fresh RPMI medium, and incubated or not with 120 µmol/L potassium antimony(III) tartrate for 12h. The preparation for 13C analysis was done by growing the parasites in RPMI where L-arginine was substituted by 13C L-arginine for 14h. -------------------------------------------------------------------- Investigated group : - ST: susceptible strain treated. - SNT: susceptible strain non–treated (controls). - R: resistant strain to Sb3+. - QC: Quality Controls samples were prepared by pooling equal volumes of each sample from all groups. --------------------------------------------------------------- Techniques: - CE-ESI-MS: CE (7100 Agilent Technologies), TOF (6224 Agilent Technologies). - LC-ESI-MS: LC (1200 series, Agilent Technologies), QTOF (6520 Agilent Technologies). - GC-EI-MS: GC (Agilent Technologies 7890A), Q (Agilent Technologies 5975) |
| URI : | http://hdl.handle.net/10637/6882 |
| Derechos: | http://creativecommons.org/licenses/by-nc-nd/4.0/deed.es |
| Fecha de publicación : | 5-feb-2015 |
| Centro : | Universidad San Pablo-CEU |
| Aparece en las colecciones: | Facultad de Farmacia |
Los ítems de DSpace están protegidos por copyright, con todos los derechos reservados, a menos que se indique lo contrario.